Decomposition of Magnesium Nitrate
2Mg NO 3 2 2MgO 4NO 2 O 2. Chemical Properties of Magnesium Nitrate.

Z Is An Anhydrous Compound Of A Group 2 Element When It Is Heated Z Undergoes Thermal Decomposition To Produce Two Different Gases Z Has Relatively Low Thermal Stablity Compared To Other
Mixtures with phosphorus tinII chloride or other reducing agents may react.

. The only other cation usually found in these salts is magnesium and it occurs as nitrate contributing to formation of NOx. Nowadays the most matured thermal energy storage TES technology for Concentrated Solar Power CSP plants is the use of molten solar salts 60 wt NaNO 3 40. Mixtures of MAGNESIUM NITRATE with alkyl esters may explode owing to the formation of alkyl nitrates.
At high pressures the stability and formation of new. Thermal decomposition of the sample followed the first-order law. Answer 1 of 3.
Magnesium nitrate has a melting point of 362 degrees Celsius. In this video we will balance the equation MgNO32 MgO NO2 O2 and provide the correct coefficients for each compoundTo balance MgNO32 MgO NO2. Effect of the impurity Magnesium nitrate cas 10377-60-3 in the thermal decomposition of the solar salt.
Check the balance The thermal decomposition of nitrate magnesium to produce magnesium oxide nitrogen dioxide and. The magnesium nitrate waste liquor can be transformed into magnesium nitrate hydrate MNH by evaporation concentration and cooling crystallization. Therefore this paper studies the effect the impurity.
Magnesium nitrate has a density of 23gcm 3. 2 9 The equation for the decomposition of magnesium nitrate is shown. 2MgNO 3 2 s 2MgOs 4NO 2 g O Which volume of gas is produced when 01 moles of magnesium.
The diffraction peak data of the aged composition match with the reference pattern number 00-004-0770 01-079-2056 and 00-044-1482 for magnesium sodium nitrate. Furthermore Magnesium-sodium nitrate illumination flares with HTPB resin have been studied for luminous efficiency by. Thermal decomposition of magnesium nitride gives magnesium and nitrogen gas at 700-1500 øC.
You are to plan a single experiment to confi rm that the molar quantities of magnesium oxide nitrogenIV oxide and oxygen produced agree with the equation for the thermal decomposition. Any nitrate in which the metal is in divalent or trivalent state generally decomposes on heating to its oxide solid residue nitrogen dioxide and free oxygen.

How To Balance Mg No3 2 Mgo No2 O2 Magnesium Nitrate Youtube
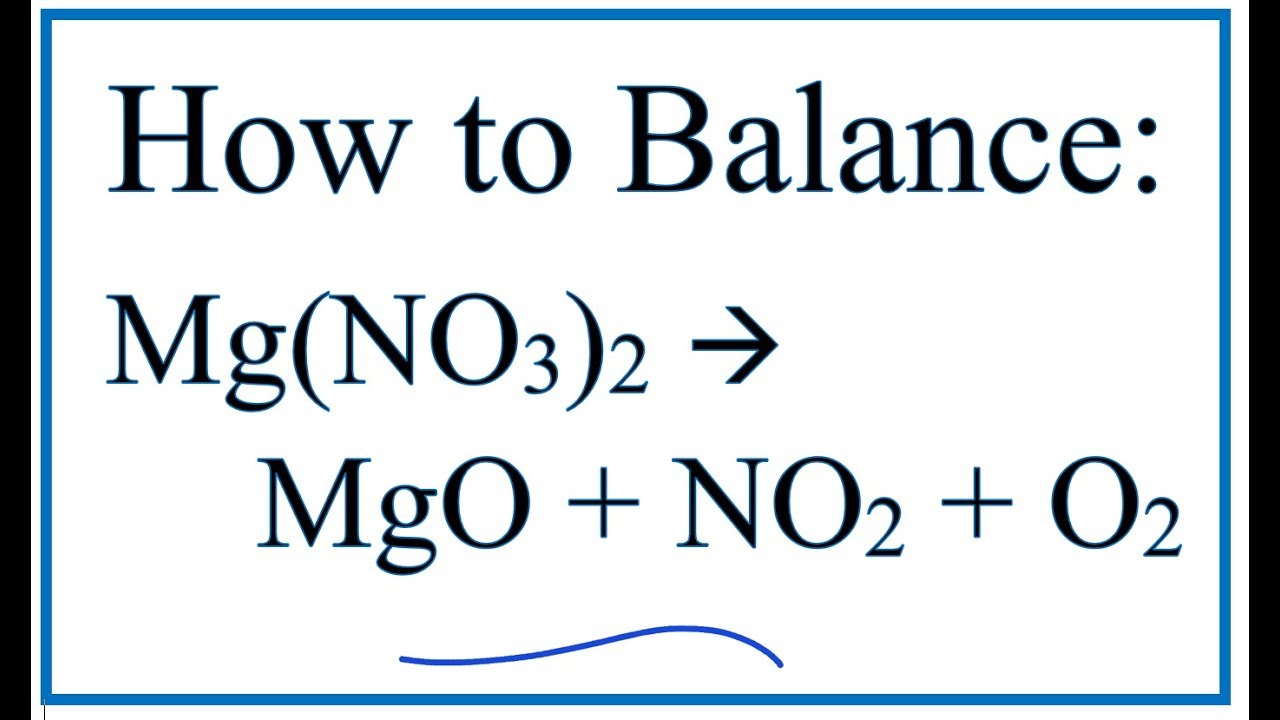
How To Balance Mg No3 2 Mgo No2 O2 Magnesium Nitrate Youtube


Belum ada Komentar untuk "Decomposition of Magnesium Nitrate"
Posting Komentar